Every flu season I hear someone say, “I received my flu shot, but I’m still sick!” Usually it is simple crankiness caused by not feeling well, but often others will comment they skip the cheap or free flu vaccine every year because they do not want to waste time on an ineffective vaccine. Sound like someone you know? Maybe even you?
Hold up there overreact-er. Vaccination is what made influenza (the scientific name for the flu) a seasonal annoyance. Before you take the path of least disease resistance, read about why you should vaccinate, how the flu vaccine is made, and what you should do with your new knowledge. The flu shot is not a panacea, but your flu symptoms are not likely caused by a bad flu shot.
Why Get Vaccinated
Vaccination is one of the biggest public health victories of the 20th century. The influenza vaccine was developed through the work of a variety of folks including Thomas Francis Jr., Jonas Salk (who would go on to develop the polio vaccine), Wilson Smith and Macfarlane Burnet. It was introduced to the U.S. Armed Services in 1942 and was licensed for the general public in 1945. The vaccine has great personal and societal benefit as it has been shown to:
- Help keep people from getting sick with the flu (i.e. what people traditionally think of when getting vaccinated).
- Reduce the risk of flu-associated hospitalization (especially important for children and older adults).
- Aid prevention for people with chronic health conditions; such as lowering rates of cardiac events among people with heart disease, and reducing hospitalizations among people with diabetes or chronic lung disease.
- Protect women during and after pregnancy.
- Make illness milder if a person gets sick with the flu.
- Promote herd immunity.
| I had a little bird, Its name was Enza. I opened the window, And in-flu-enza. -Children’s Rhyme, 1918 |
The influenza virus can be particularly deadly (see 1918-19), and influenza’s relegation to seasonal nuisance illness is largely due to the flu shot. Due to its effectiveness, there have been approximately 146 million doses of influenza vaccine distributed so far for the 2016-2017 flu season in the U.S. with somewhere in the neighborhood of 157 to 168 million projected total doses.
Yet why do folks vaccinated for influenza still get sick? It starts with how the vaccine is made.
How Is The Flu Vaccine Made?
Vaccination against influenza is an example of global cooperation. More than 100 national influenza centers in over 100 countries conduct year round surveillance to stay on top of influenza. Twice a year, the World Health Organization (WHO) convenes a group to review all available data and recommend specific strains (usually three or four) for inclusion in influenza vaccines. Some considerations are (1) which influenza viruses are making people sick, (2) the extent to which those viruses are spreading, and (3) how well the previous season’s vaccine protects against those viruses. The February meeting makes vaccine recommendations for the upcoming Northern Hemisphere’s influenza season and the September meeting makes recommendations for the Southern Hemisphere’s vaccine. The long lead-time before traditional flu season is because it takes at least six months to produce large quantities of influenza vaccine.
WHO Prepares Flu Vaccine Virus
After recommending specific strains for inclusion in influenza vaccines, the WHO prepares those strains for manufacturers to produce vaccine. This starts with the WHO creating a vaccine virus, a hybrid of a standard laboratory virus strain and the circulating virus. The hybrid contains the inner components of the laboratory strain, and the outer components of the pandemic strain. A hybrid virus takes roughly three weeks to prepare.
After preparation, the hybrid virus is verification tested to ensure the outer components create the correct proteins. The hybrid virus is now safe for use in a vaccine. Verification takes roughly another three weeks.
During creation of the vaccine virus, WHO Collaborating Centers produce reagents to enable vaccine manufacturers to measure the volume of virus they are producing and ensure packaging of the correct dosage. This takes at least three months.
Flu Vaccine Manufacture Techniques
The vaccine virus is now distributed to vaccine manufacturers. In the United States, flu vaccine is manufactured three different ways.
Fertilized Eggs
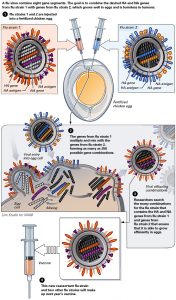
Via NIAID (Click for full size)
The predominant way of manufacturing the influenza vaccine worldwide is through injecting live influenza virus into, and growing the virus in, fertilized chicken eggs.
First, the vaccine manufacturer tests different growth conditions in their eggs to find optimal conditions. This process requires roughly three weeks. Then the vaccine virus is injected into thousands of nine to twelve-days old fertilized chicken’s eggs, which are incubated for two to three days. The bio-manufacturer then harvests the now millions of vaccine viruses from the egg white, kills it, and removes the virus protein (antigen). Antigen is the active ingredient in the vaccine, and the manufacture mixes the year’s different influenza antigens together to make the combined vaccine. This process requires about one egg per dose of vaccine requiring both a lot of healthy chickens to lay eggs and has a lot of lead time (five to six months) to mass produce.
Cell Lines
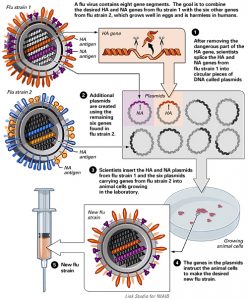
Via NIAID (Click for full size)
Flu virus is also grown in cell lines. In cell line manufacture the virus is inoculated into a mammalian cell line rather than a fertilized chicken’s egg, allowed to incubate, and then harvested. Cell grown viruses have a shorter start-up period due to the fact that the cell line is kept frozen and therefore readily available for vaccine production. In the event of a pandemic a cell line could produce vaccines faster. Cell lines are also not dependent on an egg supply for manufacture. Cell line vaccine production still involves WHO vaccine viruses grown in eggs.
Recombinant Technology
The third, newest, way to produce flu vaccine is recombinant technology. Manufacturers isolate the protein in the flu virus that induces a human immune response (the antigen), combine the protein with insect cells (hence the name “recombinant”) and replicate. In recombinant technology only the antigen, not the entire virus, is replicated so there is no risk of infection through the manufacturing process. Recombinant technology is also not dependent on an egg supply for manufacture, and currently is the only 100% egg-free vaccine on the U.S. market. So those of you allergic to eggs, take note.
Ensure Quality Control
Regardless of manufacture method, quality control and distribution are similar. Each batch of vaccine is tested and the sterility of bulk antigen verified. This can only begin once WHO laboratories supply the reagents for testing the vaccine. This process takes two weeks. The vaccine batch is diluted to the desired concentration of antigen, put into vials or syringes, and labeled. Then, random doses are tested for sterility, to confirm the protein concentration, and, for safety, by testing in animals. This process takes two weeks.
For more information on flu vaccine manufacture check out the CDC’s primer and the WHO‘s briefing note.
Why Did I Still Get Sick?
Sorry to hear you are not feeling well; please stay home to avoid spreading your infection. Yet before you shake your fist at science and swear off vaccination (see benefits above) consider these misconceptions around the flu vaccine.
Poor Timing of Illness
- You may not have the flu. The “common cold” – those assorted viruses we lump together when generically ill – has similar symptoms. The common cold is not influenza and is therefore unaffected by the influenza virus.
- You many not be fully vaccinated yet. It takes about two weeks for protection to develop after vaccination. Protection lasts through the flu season.
Influenza is a Family, Not an Individual
- Your influenza strain might not be in the vaccine. There are multiple strains of flu virus. A new flu vaccine is made each year to protect against the three (“trivalent”) or four (“quadrivalent”) viruses most likely to spread and cause illness. If you have the flu, your strain may not have be in this year’s vaccine.
- Flu vaccine varies in effectiveness each year. There are a variety of factors that play into a how effective the flu vaccine is each year, such as the person being vaccinated (their age and health) and the match between the flu viruses in the vaccine and the flu viruses spreading in the community. CDC research has shown the flu vaccine reduces the risk of flu illness by about 50% to 60% among the overall population when the virus and vaccine match well. Vaccine manufacture also plays a role. Growing the flu in eggs for one particular flu strain (A H3N2) can lead to “egg-adapted changes” and may reduce potential effectiveness against circulating viruses. Cell line and recombinant grown vaccines do not result in egg-adapted changes and may result in more effective vaccines because they are more “like” wild-type circulating viruses. This is one way the CDC is working to get around the shortcomings of vaccine manufacture.
Wisconsin Connection: The Marshfield Clinic Research Foundation based in Marshfield, WI is one of five sites around the country the CDC collects data from to measure influenza vaccine effectiveness. The Marshfield Clinic was the first site the CDC chose to collect data.
One Shot vs. the Virulent World
- Vaccines are dependent on heard immunity to prevent disease. The lower the vaccination rate the greater likelihood of exposure. If you are routinely exposed – for example you have a highly public job like teacher or healthcare worker, or come in contact with those prone to illness (i.e. you are a parent) – you are more likely to become ill regardless.
- The vaccine also helps decrease symptoms. If you had not been vaccinated you may have had more severe symptoms.
You vaccinated yourself and still became sick. What to do in the future?
Take Influenzal Action
First, continue to vaccinate yourself against influenza.
The single greatest tool we have in combating and controlling influenza is the influenza vaccine. If you do not vaccinate yourself you cannot receive the benefits of the vaccine nor aid in benefiting your neighbors and community.
Next, advocate for greater investment in vaccine research and development.
Our vaccine infrastructure could use a refresh and drug companies business models do not comparatively invest a lot in vaccines because the profit margins are lower. (Think one vaccine a year vs. a pill every month.) Does it bother you that you potentially became sick with an influenza strain not in the vaccine? We can do better. Does it bother you that the flu vaccine varies in effectiveness each year? We can do better.
Public investment in vaccine infrastructure and vaccine R&D is vital to maintaining our health as a nation, yet the proposed American Health Care Act (aka Ryancare) would cut the federal vaccines program in half. This is unfortunate because a universal flu vaccine – a vaccine that would protect a person against all strains of influenza for a lifetime – is potentially within our grasp. We need more investment even without the potential silver bullet flu shot.
The flu shot is our best tool to fight influenza. Use it.